Engineering Enzyme Stability and Resistance to an Organic Cosolvent by Modification of Residues in the Access Tunnel
Authors
Koudelakova, T., Chaloupkova, R., Brezovsky, J., Prokop, Z., Sebestova, E., Hesseler, M., Khabiri, M., Plevaka, M., Kulik, D., Kuta Smatanova, I., Rezacova, P., Ettrich, R., Bornscheuer, U. T., Damborsky, J.
Source
ANGEWANDTE CHEMIE INTERNATIONAL EDITION 52: 1959-1963 (2013)
Abstract
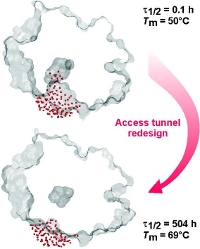 Mutations targeting as few as four residues lining the access tunnel extended enzyme’s half-life in 40% dimethyl sulfoxide from minutes to weeks (4,000-fold) and increased its melting temperature by 19 °C. Protein crystallography and molecular dynamics revealed that the tunnel residue packing is a key determinant of protein stability and the active-site accessibility for co-solvent molecules (red dots). The broad applicability of this concept was verified by analyzing twenty six proteins with buried active sites from all six enzyme classes.
Mutations targeting as few as four residues lining the access tunnel extended enzyme’s half-life in 40% dimethyl sulfoxide from minutes to weeks (4,000-fold) and increased its melting temperature by 19 °C. Protein crystallography and molecular dynamics revealed that the tunnel residue packing is a key determinant of protein stability and the active-site accessibility for co-solvent molecules (red dots). The broad applicability of this concept was verified by analyzing twenty six proteins with buried active sites from all six enzyme classes.














